Is your wire stripping process holding back your validation efforts? In medical device assembly, tweezers are still widely used to strip insulation from fine wires, especially in catheter builds, neurostimulation leads, and pacing systems. They’re simple, familiar, and cost-effective. But when it comes to meeting the rigorous demands of FDA, ISO, and GMP validation, manual methods introduce more risk than reliability. Variability, lack of traceability, and operator dependency make tweezers a weak link in an otherwise tightly controlled process.
That’s where laser wire stripping comes in Not just as a modern replacement, but as a validation-ready solution built for precision, repeatability, and full process control. And with proven adoption across medtech manufacturers, the switch can be made confidently without disrupting compliance.
Why Manual Tweezers Fail in a Validation Context
Tweezers are frequently used for stripping fine wires in assembly lines for catheters, neurostimulation leads, and pacing systems. While operators may achieve acceptable results in small batches or R&D settings, several challenges arise when validation is required:
-
Lack of repeatability: Manual stripping is inherently variable. Strip lengths can fluctuate, conductor damage can occur, and operator skill plays an outsized role.
-
No process traceability: Tweezers can’t log or monitor their output. Auditors and quality managers are left with training records rather than hard data.
-
Poor scalability: As volumes grow or new operators are added, consistency becomes harder to maintain, leading to rework, scrap, and validation drift.
This creates a serious problem when trying to validate a process under ISO 13485, FDA 21 CFR Part 820, or even during internal GMP audits.
Laser Wire Stripping: Precision That’s Built for Validation
Laser stripping systems provide a non-contact, programmable, and highly repeatable alternative that lends itself naturally to medical-grade validation protocols.
1. Cleanroom Qualification
Modern laser wire stripping systems can be installed and operated in ISO Class 7 and 8 cleanrooms, provided the appropriate fume extraction and safety protocols are in place.
-
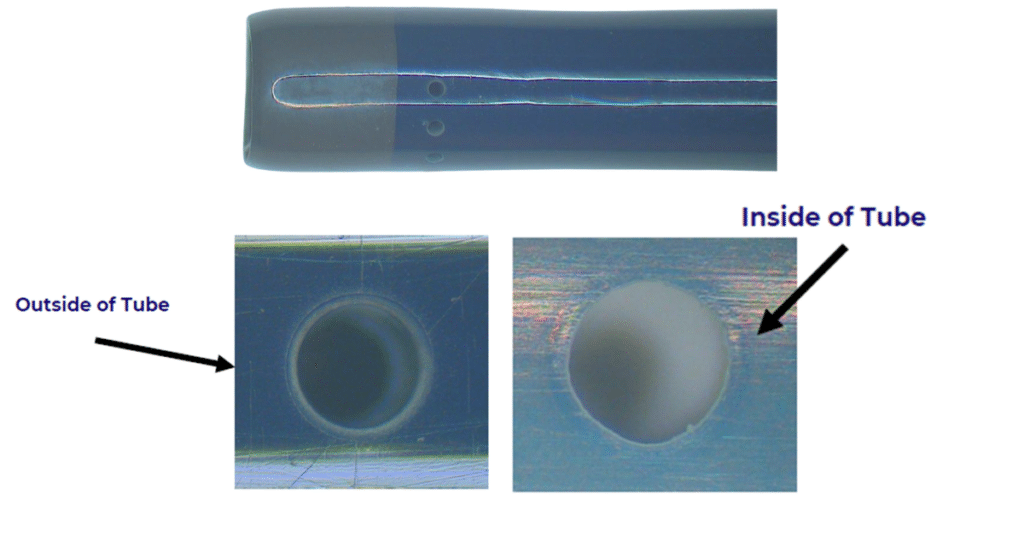
Particle-free process: Unlike tweezers or mechanical blades, lasers don’t generate physical debris or burrs. Any vaporized material is removed via fume extraction, reducing the risk of foreign object debris (FOD).
-
Compact, enclosed designs: Many systems are built with minimal moving parts and sealed optics, making them suitable for sterile or semi-sterile environments.
-
Material compatibility: Medical-grade wires, including PTFE, ETFE, and polyimide-insulated conductors, can be stripped without nicking or scorching, critical for devices where failure is not an option.
2. Documentation Packages and FAT/SAT Support
Laser system suppliers familiar with the medical device sector typically offer comprehensive validation support:
-
Factory Acceptance Testing (FAT): Before shipment, systems are tested using customer-supplied wires or documented test protocols to confirm process repeatability, stripping accuracy, and safety interlocks.
-
Site Acceptance Testing (SAT): After installation, the same testing standards can be replicated to confirm the system performs as expected in the production environment.
-
DQ/IQ/OQ documentation: Laser suppliers often provide templated or custom documentation for Design Qualification (DQ), Installation Qualification (IQ), and Operational Qualification (OQ), speeding up the validation timeline.
-
Software traceability and recipe control: Parameters like laser power, scan pattern, and number of passes can be locked, logged, and controlled, eliminating undocumented process drift.
This contrasts sharply with manual methods, where validation relies heavily on subjective operator skill and visual inspection.
3. Real-World Adoption: From Catheters to Neuromodulation
The transition from tweezers to lasers isn’t theoretical. It’s already happening in leading-edge medical device companies.
Catheter Manufacturers:
One U.S.-based catheter OEM replaced manual wire prep with a laser process to reduce insulation residue and avoid dielectric breakdown in micro magnet wire. Not only did this improve yield, but it also helped them pass a critical customer audit with zero non-conformances.
Neurostimulation Device Firms:
For neuro leads with ultra-fine wires (often 42 AWG and smaller), lasers allow precise ablation of insulation without damaging delicate conductors, something that’s nearly impossible with tweezers or blades. The process is easily validated thanks to tight control over pulse energy and beam path.
Cardiac Rhythm Management (CRM):
Pacing and defibrillator lead manufacturers often deal with fine, multi-conductor cables in tight form factors. Manual stripping introduces unacceptable risk of nicks or inconsistent strip lengths. Switching to laser systems enabled these firms to meet OQ performance specs and demonstrate repeatability across thousands of cycles.
Replace Tweezers, Gain Confidence
Laser wire stripping isn’t just about speed or sophistication, it’s about confidence. Confidence that the process won’t drift. Confidence that the data is traceable. Confidence that the next FDA or ISO audit will pass without scrambling for subjective inspection logs.
For medical device manufacturers looking to validate every critical process step, laser wire stripping provides a low-risk, high-reward alternative to tweezers. And for those ready to make the switch, the path to validation is already paved.
Next Steps:
Want to learn how your team can validate a laser stripping process for your specific application? Contact us today.